
Search

Basic Background and History of PAT
The Process Analytical Technology (PAT) initiative originated from the 2004 guidelines issued by the U.S. Food and Drug Administration (FDA) as a system for designing, analyzing, and controlling manufacturing processes through the timely measurement of critical quality attribute (CQA) in raw materials and processes and key performance indicators (KPI), to realize the automatic control within predefined limits for the objective of ensuring the quality of the end product of the process, i.e., "product quality by design and process" rather than by the endpoint detection.
On-line process analyzers are typical PAT tools, whose output is used for the analysis of various scales, with the nature of controlling the manufacturing process to achieve QbD. PAT is a money-saving tool in the long run and does not bring new specific requirements needed to support cGMP approval.
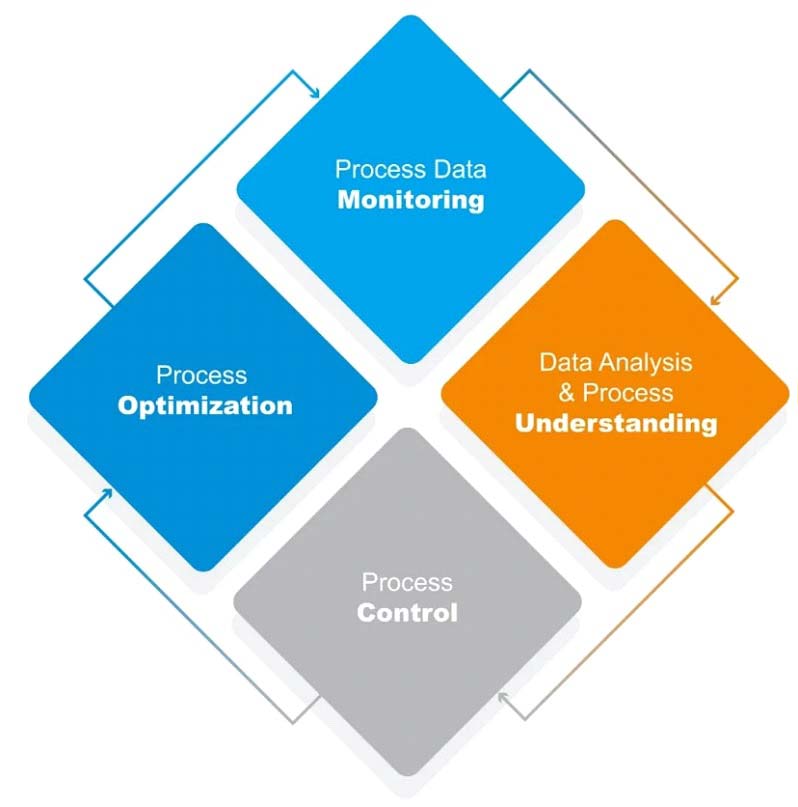
The role of PAT in biopharmaceutical manufacturing process is divided into four processes: process data monitoring, data analysis and process understanding, process control and process optimization. Through the analysis and optimization of process data, the process stability is achieved to realize the process monitoring of antibody drugs, cell drugs and other biological drugs.
Cell Culture Bioreactors and PAT Configuration
Bioreactors for animal cell culture are generally constructed with modular design.
- CFD designed tank and stirring system
- PAT monitoring system with multi-scale parameters monitoring
- Four-gas automatic control
- Tank weighing and replenishment weighing system
- SIP fully automatic sterilization
- CIP on-line cleaning
- Automatic tank pressure control system
- Multiple aseptic sampling
- Lower computer control and data analysis software
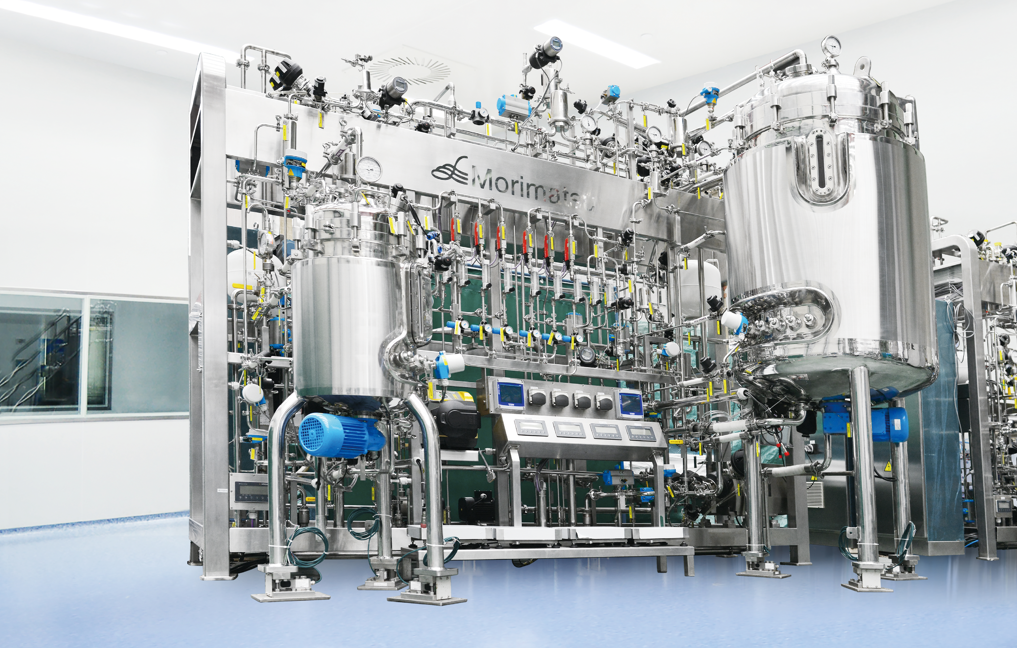
At present, there are not many on-line monitoring sensors with PAT installed on bioreactors at home and abroad, the main reason of which is the on-line technology is still not mature enough.
Cell Culture Parameter Detection And The PAT Role
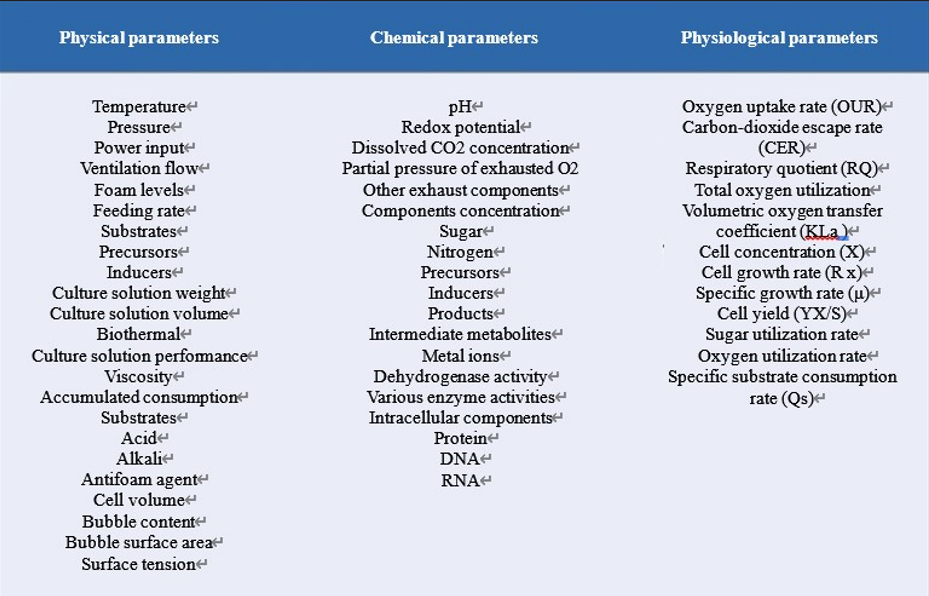
Process parameters are the key basis for cell culture or microbial fermentation process control. The detection of cell process parameters is divided into two methods: one is to use the sensor for on-line detection and analysis; the other is to take out samples from fermentor or bioreactor for off-line detection. The parameters obtained from the former method are called On-line parameters, and those obtained from the latter method become Off-line parameters. All obtained parameters are mainly classified into physical, chemical and physiological parameters. There are also engineering parameters if amplification is required. See the following table for details:
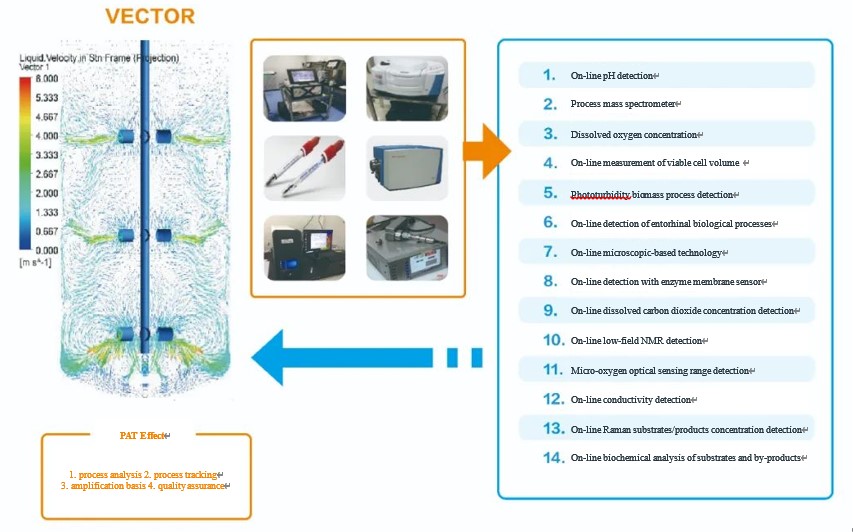
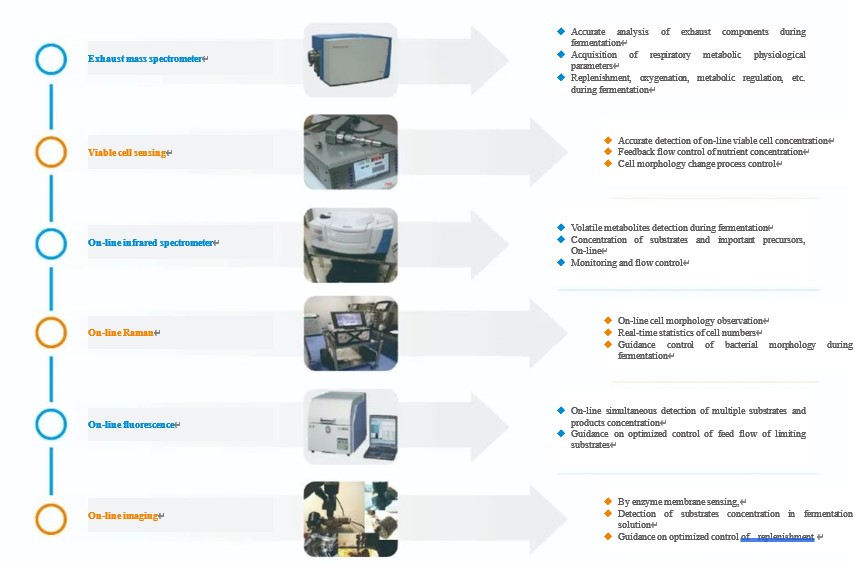
PAT has a role in process analysis, process tracking, amplification benchmarking and quality assurance in cell culture applications. However, the role for each on-line analytical instrument has its own biological significance (As shown in the figure below). The multi-parameter correlation analysis provides insights into the physiological state of cell growth, thus providing optimization and control of the process.
PAT Development Trend
With the development of on-line sensor technology and big data technology, the biopharmaceutical industry embraces digitalization and automation technology and develops networked big data and intelligent technology, which will overturn the current production status quo of manual-based operation, analysis, decision-making production and enter the intelligent era of Internet of Everything. For the development of biopharmaceuticals in the next 5–10 years, there will be huge developments in the following two areas.
1. PAT Parameters And Process Digitalization Technology
Integration of no less than 10 on-line monitoring devices, including conventional sensors, process analysis sensors and cell in-situ analysis systems, etc.; integration and consolidation of integrated cross-dimensional multi-scale parameters to form a digitalized process management system, to realize the interchange of real-time detection data, off-line parameters, images (videos) information of reactors and analytical instruments, etc., and to form a biological process big data detection system as the data basis for artificial intelligence decision-making.
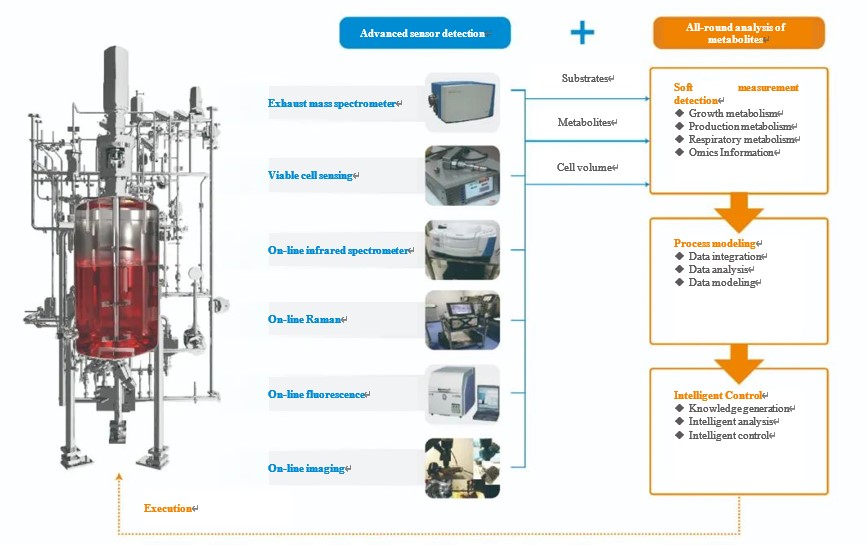
2. Innovative Primary Artificial Intelligence Control Process
Based on the deep knowledge of biological processes and the construction of large databases, a global real-time on-line analysis system for the cell culture process will be created through the current manually experienced cell culture process, and a knowledge map and digital twin system for cell metabolism will be constructed to form a production optimization process based on the dynamic regulation of cellular processes in a multidimensional cellular metabolism model.
As the leading company in the field of bioreactors in China, Morimatsu will continue to focus on the development of PAT and bring the latest design concepts and the best quality products to our customers. At the same time, Morimatsu will also continue to lead the industry with its cutting-edge engineering philosophy and advanced construction methods, and will combine its own characteristics to form a unique brand competitiveness and move steadily towards more diversified sectors.
About Morimatsu Life Science
Morimatsu Life Science is the sanitary business segment of Morimatsu International Holding Co., Ltd. (Morimatsu International, stock code: 2155.HK), covering industries such as pharmaceuticals, biopharmaceuticals, FMCG, cosmetic and electronic chemicals, and mainly includes Shanghai Morimatsu Pharmaceutical Equipment Engineering Co., Ltd., Morimatsu (Suzhou) Life Technology Co., Ltd., Shanghai Morimatsu Biotechnology Co., Ltd., Pharmadule Morimatsu AB (Sweden) and other companies and their affiliates, focusing on the research and development, manufacturing and sales of products in related fields. Morimatsu, founded in Japan and taken root in China, has developed into a multi-national company that embraces globalization, masters core technology, and gains rich experience from project implementation in diverse fields including core equipment, process systems, and engineering solutions. Starting from its advanced manufacturing base in China, Morimatsu International has opened subsidiaries in Sweden, the United States, India and Italy, and has delivered different forms of products and services to more than 40 countries and regions so far, by virtue of its global footprint of efficient and professional team.
Forward-Looking Statements
The information in this press release may include some forward-looking statements. Such statements are essentially susceptible to considerable risks and uncertainties. The use of “predicted”, “believed”, “forecast”, “planned” and/or other similar words/phrases in all statements related to our company is to indicate that the statements are forward-looking ones. Our company undertakes no obligation to constantly revise such predicted statements.
Forward-looking statements are based on our company management’s current perspectives, assumptions, expectations, estimations, predictions and understanding of future affairs at the time of the making of such statements. Such statements are not guaranties of future development and are susceptible to the impact of risks, uncertainties and other factors; some are beyond the control of our company and unpredictable. Subject to the influence of future changes and development in our business, competition environment, political, economic, legal and social conditions, the actual outcomes may differ significantly from the information contained in the forward-looking statements.