01 Abstract
Adjuvant, also known as an immunomodulator or immune booster, enhances the body’s immune response to an antigen or changes the type of immunity. Early in the 1920s, an abscess developed at the inoculation site when Ramon inoculated horses with diphtheria toxin, where the specific antibody titer was at a high level. It was later discovered that the abscess was caused by injection of an unrelated substance that increased the immune response against the toxoid, leading to the concept of “adjuvant”. Since then, as new vaccine technologies have matured, new vaccines such as recombinant protein vaccines, cleaved vaccines, and subunit vaccines have emerged, giving a great impetus to the development of modern novel adjuvants.
Currently approved adjuvants for human vaccines include aluminum salt-based adjuvants such as aluminum hydroxide and aluminum phosphate, oil-in-water emulsions such as MF59 and AS03, MPL (monophospholipid A)-based adjuvants such as AS04 and RC-529, and influenza virus virosome (Table 1).
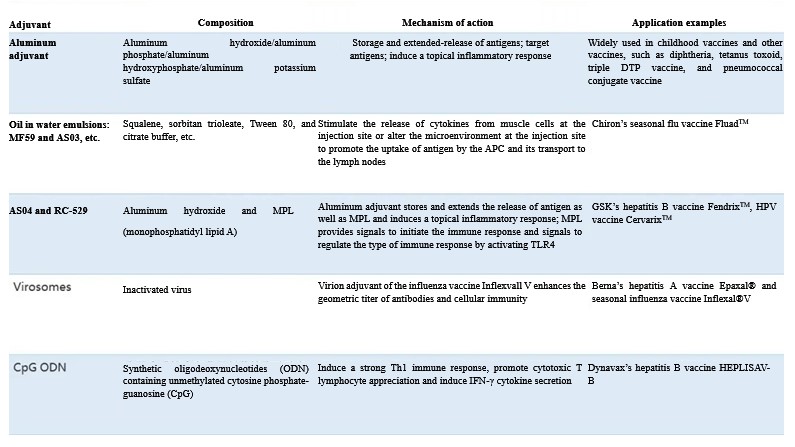
Table 1 Statistics of marketed vaccine adjuvants
Aluminum salts have been used as vaccine adjuvants for almost 90 years. and even today they are also still the only adjuvants approved for widespread use in human vaccines. Aluminum adjuvants play an important role in response to outbreaks of infectious diseases and in the development of novel vaccines because of their relatively well-defined mechanism of adjuvancy, safety, efficacy and relatively good tolerability. Aluminum adjuvants will remain the most widely used for human vaccines for a long time to come and will be a key weapon for new vaccines to stand out!
02 Classification and characteristics of aluminum adjuvants
Aluminum adjuvants mainly include aluminum phosphate [Al(OH)x (PO4)y], aluminum hydroxide [Al(O)OH] and potassium aluminum sulfate [KAl(SO4)2-12H2O], and the first two are currently the most commonly used.
Aluminum adjuvants all contain hydrophilic groups on their surface, such as hydroxyl, oxygen or phosphate groups, and the charges on their surface are determined by pH.
Aluminum hydroxide is a layered crystal with only hydroxyl groups on its surface, which behaves as an amphoteric compound because its hydroxyl groups can provide or receive protons. It exists as a cation in pH 7.4 solution and is a good adsorbent for anionic antigens.
Aluminum phosphate adjuvant is a hydroxyaluminum phosphate complex in an amorphous structure. it is a non-stoichiometric compound, and there is no fixed ratio between hydroxyl and phosphate. The displacement of the hydroxyl group by the phosphate group depends on the conditions of the reactants and precipitation and their isoelectric points. It exists as an anion in solutions at pH 7. 4 and is a good adsorbent for cationic antigens.
Different aluminum salts have different isoelectric points, for example, the isoelectric points of aluminum hydroxide and aluminum phosphate are 11.4 and 5, respectively. Protein antigens (especially acidic protein antigens) and virus-like antigens are in general mostly adjuvanted with aluminum hydroxide, for example, aluminum hydroxide is widely used as an immune adjuvant in hepatitis B and triple DTP vaccines. A small number of protein antigens and proteoglycan-conjugated antigens use aluminum phosphate adjuvant. For example, aluminum hydroxyphosphate is used as an immune adjuvant in polyvalent conjugate vaccines for the prevention of pneumococcal disease.
03 Mechanism of action of aluminum adjuvants
Although aluminum adjuvants have been used in human vaccines for many years, their mechanism of action is complex, as evidenced by the fact that aluminum adjuvants often activate many immune response chains at the same time, and it is difficult to analyze the effect of reaction chains related to antigen-specific immune response separately. In terms of effect, it may involve the following roles: (1) storing and diluting antigens: aluminum adjuvants after adsorbing antigen increase the surface area of antigens, fully expose the antigen surface binding site, extend the release of antigens, improve antibody response, enhance the immunogenicity of antigens, stimulate antigen presentation, promote cytokine secretion, strengthen the systemic action of T and B cells, and continuously stimulate immune cells over a long period of time, thus maintaining an effective immune response process and ultimately producing good immune memory. (2) Targeting antigens: manoparticles containing both aluminum adjuvants and antigens are more likely to activate B cells by cross-linking BCR, and more importantly, they are more likely to target antigen-presenting cells with phagocytosis. (3) Inducing topical inflammatory response: aluminum adjuvants are able to induce sterile inflammation at the injection site, as vast quantities innate and acquired immune cells are recruited to the injection site and adjacent draining lymph nodes, where a large number of these cells mediate the subsequent vaccine antigen-specific immune response.
04 Difficulties and solutions in aluminum adjuvant production
1. Raw materials for production and quality control
Aluminum adjuvants are prepared using inorganic salts as raw materials, which in principle should comply with the provisions of the “Quality Control Procedures for Raw Materials and Excipients for the Production of Biological Products” in the Chinese Pharmacopoeia.
2. Preparation and production process
Rigorous design of quality attributes and process parameters is an essential prerequisite for process development. The development of aluminum adjuvants can be performed in accordance with ICH Q8[1] and other internationally accepted principles, to determine the process route, process parameters and define the process control strategy based on the candidate key quality attributes of the aluminum adjuvants and/or vaccines.
The particle size and consistency of the particle size distribution of aluminum adjuvants, as well as the high adsorption capacity for antigens, are generally considered as important indicators of product quality and stability in the preparation process, which are also major factors influencing the immune response of the organism[2]. Smaller aluminum adjuvant particles produce a larger specific surface area, resulting in greater surface reactivity as well as a higher rate of antigen adsorption, thus ensuring better antigen extraction, transport and interaction with the antigens[3]. Studies have shown that mice also better tolerate aluminum phosphate with a smaller particle size.
Less than 10 μm is generally considered to be an optimized particle size. The current aluminum hydroxide adjuvant Alhy-drogel® commercially available has a particle size of 3.07 μm, and the aluminum phosphate adjuvant Adju-phos® has a particle size of 4.26 μm.
Based on the considerations above, the focus should be placed on the following preparation parameters such as reaction temperature, reaction pH control, stirring control, feeding method of reaction materials, ultrafiltration and liquid replacement, and sterile processing during the aluminum adjuvant production as shown in Figure 1.

01 Reactant selection
There are 2 ways to prepare Al(OH)3 adjuvant. One is the ammonia method, where aluminum trichloride (AlCl3) reacts with ammonia to produce Al(OH)3 colloid. The other is to have aluminum trichloride react with sodium hydroxide to produce Al(OH)3. The latter is more commonly used in production. The study shows that the preparation process of aluminum chloride plus sodium hydroxide is simple, easy to control the process parameters and adjuvant quality, and has a better effect on vaccine efficacy enhancement than the formulation of aluminum trichloride plus ammonia[4] . In contrast, the preparation of aluminum phosphate adjuvant requires the reaction of aluminum salt solution with phosphate solution system, and the concentration ratio of reactants should be precisely controlled during the reaction.
02 Reaction temperature control
Studies have shown that the Al(OH)3 colloidal particles were unstable and prone to large particle precipitation after subsequent autoclaving when the reaction occurred at 58-62°C, while the Al(OH)3 colloidal particles were stable with good turbidity after high temperature setrilization when the reaction occurred at 70-72°C[5]. The results of the particle size distribution of the resulting adjuvants when preparing aluminum phosphate adjuvant at different mixing temperatures showed that the particle size of the aluminum phosphate particles prepared at 75°C was slightly larger than that of the adjuvants prepared at 65°C, and both were significantly finer and more uniform than the adjuvant particles prepared at room temperature[6]. Therefore, we should strictly control the reaction temperature during the actual production of adjuvants. For the adjuvant reaction tank, the reaction temperature can be accurately controlled using the temperature control unit to avoid fast reaction temperature rise and high temperatures.
03 Reaction pH control
Since aluminum phosphate adjuvant is an amorphous hydroxyaluminum phosphate salt and there is no fixed ratio between hydroxyl and phosphate, pH control during precipitation affects the ratio of hydroxyl to phosphate groups. The increase in hydroxyl concentration at higher pH stimulates the hydroxyl ions to compete more effectively for coordination sites around aluminum, while the precipitation at a lower pH level favors the formation of Al-PO4 bonds instead of Al-OH bonds. In this way, the different groups will lead to changes in aggregation, and then affect the particle size. And the exposure of different groups on the adjuvant surface may also lead to large differences in the type and capacity of adsorbed proteins. Studies have shown that some antigens can be well adsorbed onto the surface of aluminum adjuvants at pH below 6 [7], and that when pH deviates from the range additional ions such as phosphate groups, amino acids, peptides, polysaccharides and other impurities will reduce antigen adsorption by competing with the antigen for adsorption sites. In general, low ionic strength, few phosphate groups and few impurities facilitate the adsorption of antigens onto the aluminum adjuvant surface. Therefore, to produce homogeneous aluminum phosphate adjuvant, pH should be kept stable during precipitation, as high pH will cause a large amount of Al(OH)3 colloid to be unstable during later sterilization and thus produce large particle precipitation, which will cause great difficulties in the subsequent liquid replacement and purification, while low pH will fail the composition and affect the performance of the adjuvant. Therefore, during the preparation of aluminum adjuvants, it is necessary to monitor the pH change at all times and ensure a stable flow rate in the material feeding, and the reaction process should be uniform to avoid too fast reaction in some parts.
04 Feeding methods
In large-scale production, the material feeding is often controlled by the “precision mass flow meter + control valve”. For small preparation systems, a highly stable diaphragm pump or a uniform flow peristaltic pump with a high-precision mass flow meter can be used for precise control. To avoid fast reaction in some parts, a custom static mixer or a dynamic mixer can be adopted before feeding the material into the tank for uniform and full reaction, which may avoid different reaction states in different parts after the material is directly fed into the tank. The material can also be fed into another reaction material by multi-point injection or multi-point dropwise addition, and the mixing rate is controlled according to the material feeding rate, so that the obtained aluminum adjuvant particles are small in size with good consistency.
05 Ultrafiltration and liquid replacement
The purpose of ultrafiltration and liquid replacement is mainly to remove free phosphate ions or aluminum ions by changing the buffer system of the aluminum adjuvant, and reduce the ionic strength, thus obtaining a high purity aluminum adjuvant. And the buffer system can enhance the adsorption of the adjuvant to the antigen by changing the isoelectric point of the adjuvant[8]. Moreover, a good buffering system allows the aluminum adjuvant to be preserved for a long time without aggregation.
06 Sterile processing
The use of an automated sterile processing system for adjuvants requires attention to the effect on adjuvant quality and particle aggregation. The entire sterile preparation system is designed to be fully hygienic. A sterile dispensing system is required for adjuvant dispensing. In addition, a comprehensive risk assessment and validation should be carried out for the control of sterility assurance in the preparation and use of adjuvants.
Based on the in-depth understanding of aluminum adjuvant product process and accurate interpretation of industry regulations, Morimatsu can provide digital intelligent overall engineering solutions including core equipment (Machines), value-added services (Values) and modular engineering (Plants) for the R&D, pilot and commercial production of aluminum adjuvants. In terms of core equipment and modular systems, Morimatsu can provide complete and comprehensive solutions, including buffer dispensing and storage systems, adjuvant reaction systems, hollow/ultrafiltration systems, and automatic adjuvant filling systems.
Morimatsu introduces the ultimate modular solution help reduce 85~95% of the workload on site. The manufacturing and implementation in Morimatsu factory allows the full use of excellent resources and professional engineers from home and abroad to solve various problems concerning the project efficiently, thus achieving high quality project delivery and improving the flexibility of project implementation.
The service coverage of the whole life cycle of Morimatsu includes front-end services such as joint R & D, process scale-up, feasibility study, basic design, conceptual design, and digital top-level design, to product production, as well as digital operation and maintenance, verification, and other back-end services after delivery, which better help to quickly realize process transfer, process scale-up, and industrialized production.
References:
1. Pharmaceutical Development, ICH Q8,2009
2. GUOTA R K. Aluminum compounds as vaccine adjuvants[J]. Adv Drug Deliv Rev,1998,32:155-172
3. CHEN Y L. Preperation of aluminum (oxy)hydroxide nanorods adjuvant and its immune effects of adsobing Pseudomonas Aeruginosa [D]. Chongqing:Chongqing University,2019. (in Chinese)
4.Ye L, Liu DY. Comparisons on the Effect of Recombinant Hepatitis B Vaccines Adjuvanted with Aluminum Hydroxide from the Two Different Prescriptions [J]. Progress in Microbiology and Immunology, 1999, 27(4): 51
5.Li DJ, He W. Optimization of Procedure for Preparation of Aluminum Hydroxide Adjuvant [J]. Chinese Journal of Biologicals, 2010, 23(10): 1136
6.Han L. Preparation of Aluminum Adjuvant and Its Application on Adsorbed Diphtheria, Tetanus and Acellular Pertussis Combined Vaccine and Protein Based Pneumococcal Vaccine [A].2015 : 26-27
7. LINDBLAD E B. Aluminum adjuvants// STEWAR-TULL D E S. The theory and practical application of adjuvants[M]. Chichester: John Willey and Sons Ltd,1995:21-35
8.Yue Z, Zhao YX. Properties and Advance in Research of Aluminum Adjuvant [J]. Chinese Journal of Biologicals, 2016, 29(12): 1352
About Morimatsu Life Science
Morimatsu Life Science is the sanitary business segment of Morimatsu International Holding Co., Ltd. (Morimatsu International, stock code: 2155.HK), covering industries such as pharmaceuticals, biopharmaceuticals, FMCG, cosmetic and electronic chemicals, and mainly includes Shanghai Morimatsu Pharmaceutical Equipment Engineering Co., Ltd., Morimatsu (Suzhou) Life Technology Co., Ltd., Shanghai Morimatsu Biotechnology Co., Ltd., Pharmadule Morimatsu AB (Sweden) and other companies and their affiliates, focusing on the research and development, manufacturing and sales of products in related fields.
Morimatsu, founded in Japan and taken root in China, has developed into a multi-national company that embraces globalization, masters core technology, and gains rich experience from project implementation in diverse fields including core equipment, process systems, and engineering solutions. Starting from its advanced manufacturing base in China, Morimatsu International has opened subsidiaries in Sweden, the United States, India and Italy, and has delivered different forms of products and services to more than 40 countries and regions so far, by virtue of its global footprint of efficient and professional team.
Forward-Looking Statements
The information in this press release may include some forward-looking statements. Such statements are essentially susceptible to considerable risks and uncertainties. The use of “predicted”, “believed”, “forecast”, “planned” and/or other similar words/phrases in all statements related to our company is to indicate that the statements are forward-looking ones. Our company undertakes no obligation to constantly revise such predicted statements.
Forward-looking statements are based on our company management’s current perspectives, assumptions, expectations, estimations, predictions and understanding of future affairs at the time of the making of such statements. Such statements are not guaranties of future development and are susceptible to the impact of risks, uncertainties and other factors; some are beyond the control of our company and unpredictable. Subject to the influence of future changes and development in our business, competition environment, political, economic, legal and social conditions, the actual outcomes may differ significantly from the information contained in the forward-looking statements.







