
Search

A Single Use System (SUS) is a process equipment solution that is typically assembled from polymeric material components to form a system or unit of operation for a single or one-stage pharmaceutical production activity. SUS should be considered for the following elements in quality control:
SUS compatibility assessment
SUS compatibility assessment involves the safety of the materials used throughout the life cycle of the SUS application, including chemical compatibility, biocompatibility, animal components, extractables and leachables, non-specific adsorption, etc.
SUS physical properties
Primarily tests for robustness and system integrity (material thickness, smoothness, flexibility and ductility, etc.), material strength, puncture resistance, and component and system integrity.
SUS raw material controls
Compliance and stability of raw material quality standards to ensure product lot-to-lot consistency.
The supply of raw materials shall ensure the continuity of SUS production, and critical quality attributes should be identified with upstream suppliers.
SUS production process control
Processes and key process parameters should be established on the basis of comprehensive validation and sound production operation specifications.
During the production process, the production process parameters are strictly enforced, the risks that may be introduced in the production are vigorously controlled, and the corresponding monitoring items and limit indicators are established.
SUS aseptic guarantee
SUS is mainly made of plastic, with complex structure, different materials and different shapes, so the evaluation of the sterilization process, verification of the sterilization process, post-sterilization evaluation, and the determination of the relevant process parameters are particularly important for the sterilization process guarantee. Factors affecting the sterilization process include the bioburden of the sterilized product, the type of microorganisms, the material, quantity, size and shape of the product, and the complexity of the SUS system.
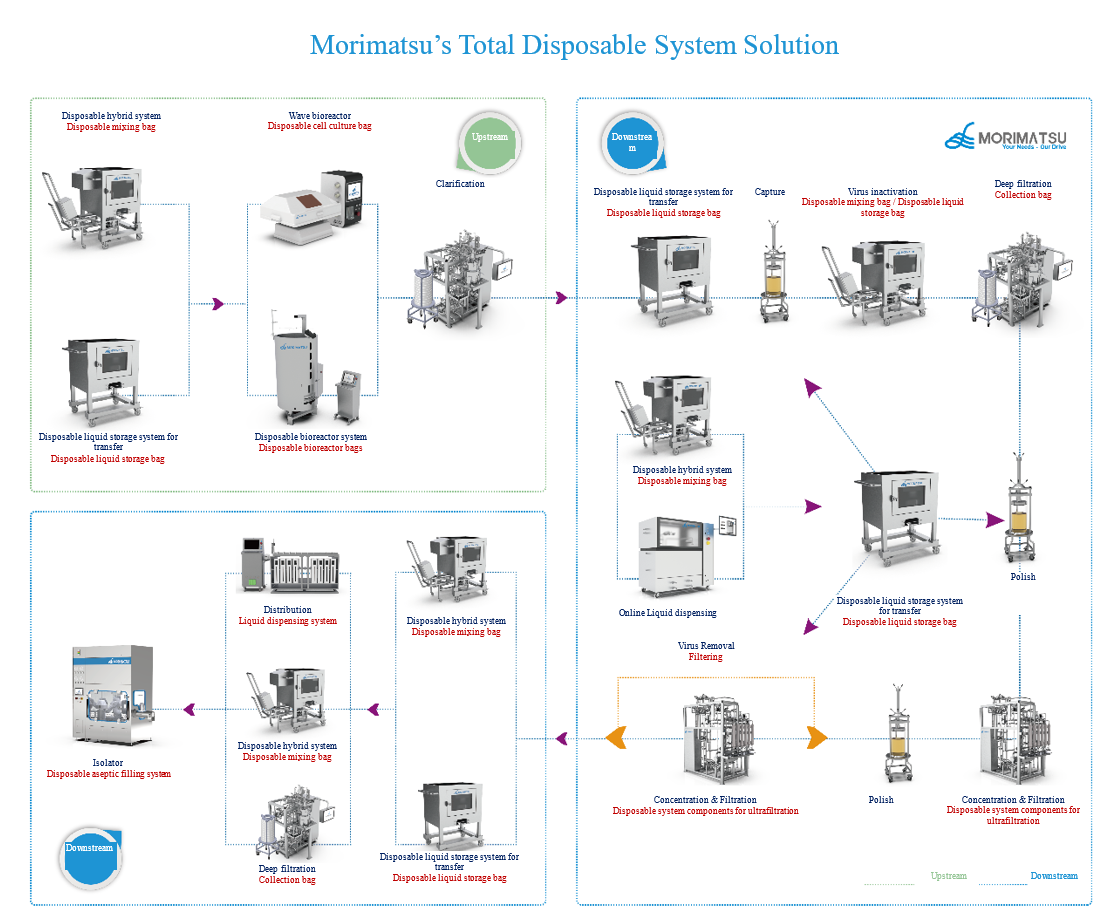
With the rapid development of pharmaceutical process equipment and the increasing maturity of single use technology, single use systems are more and more widely used in the pharmaceutical field, especially in the production of vaccines, genetically engineered products, monoclonal antibodies and other biopharmaceuticals.
Morimatsu is committed to meeting the different needs of customers in the biopharmaceutical industry at home and abroad with high-quality and reliable single use system solutions. Our product process and quality are sound and reliable. With verification tests that meet USP and other international standards, we can provide customers with abundant verification document support.
About Morimatsu LifeSciences
Morimatsu LifeSciences is a subsidiary of Morimatsu International Holdings Limited (Morimatsu International, stock code: 2155.HK), covering pharmaceutical, biopharmaceutical, FMCG, cosmetics, electronic chemicals and other industries, provides clients with "core equipment/Machinery + Value added + digitalized intelligence overall Plant Solutions and Service" (" MVP Solutions & Service"), mainly including Shanghai Morimatsu Pharmaceutical Equipment Engineering Co., LTD., Morimatsu (Suzhou) LifeSciences Co., LTD., Shanghai Morimatsu Biotechnology Co., LTD., Shanghai Mori-Biounion Technology Co.,Ltd. and Pharmadule Morimatsu AB., Morimatsu Pharmadule (Singapore) Private Limited as well as their subsidiaries. Morimatsu LifeSciences is focusing on the research and development, manufacturing, and sales of products in related fields.
From the start in Japan, Morimatsu has grown and today is a diversified multinational corporation with vast experience and professional know-how in fields of process engineering equipment as well as modular engineering solutions. Our company has established long-term collaboration relationships with numerous well-known global corporations. We have companies in Sweden, Italy, US, India, Singapore, Malaysia, together with the large scale operation and manufacturing facilities in Asia. So far, Morimatsu has delivered projects to more than 40 countries/regions in the world, established an excellent industrial reputation in the process.
Forward-Looking Statements
The information in this press release may include some forward-looking statements. Such statements are essentially susceptible to considerable risks and uncertainties. The use of "predicted", "believed", "forecast", "planned" and/or other similar words/phrases in all statements related to our company is to indicate that the statements are forward-looking ones. Our Company undertakes no obligation to constantly revise such predicted statements.
Forward-looking statements are based on our Company management's current perspectives, assumptions, expectations, estimations, predictions and understanding of future affairs at the time of the making of such statements. Such statements are not guarantees of future development and are susceptible to the impact of risks, uncertainties and other factors; some are beyond the control of our Company and unpredictable. Subject to the influence of future changes and development in our business, competition environment, political, economic, legal and social conditions, the actual outcomes may differ significantly from the information contained in the forward-looking statements.