
Search

Recently, NCPC, GeneTech successfully completed a 100% 3D engineering design review in the “Intelligent Upgrading Project of Vaccine Preparation Production Line" by adopting Morimatsu LifeSciences' VR review technology.
As early as in the design phase, the project used Morimatsu's Smart Process & Instrumentation Diagram (PID) technology and digital 3D design technology, and further adopted Morimatsu's self-developed VR review technology platform in the design review process. Morimatsu is a pioneer in the industry to apply its VR review technology platform to the review of engineering design project.
Morimatsu's VR Review Platform
Morimatsu's VR review platform includes a diverse spectrum of functions such as review report, location transfer, paintbrush, photo taking, voice entry, scene navigation, and review record follow-up.

(Login interface)

(Function menu)
In the product design stage, the 3D model can be imported into the review system to generate a VR review program, which helps the design team and customers better experience the design results, pinpoint the problems, and reduce omissions, thus minimizing rework.
Project Application
Morimatsu's digital design technology achieves a simulated workshop on a 100% scale. By wearing VR glasses, the reviewers are able to walk freely in the virtual workshop to review the design rationality of the new workshop design, including personnel operating space, personnel operating height, equipment size, equipment access, etc., and improve the efficiency and quality of the review.
In addition, NCPC, GeneTech has used Morimatsu's VR review platform to enhance smartness and efficiency in the following aspects:
- Through the first view of the person in the virtual scene, it is possible to spot problems that may otherwise be neglected during the 3D design review on the computer;
- The distance between different devices can be measured with millimeter-level accuracy;
- Making graffiti-style comment on problems found during the review is supported;
- Through Text entry via voice recognition and photographic evidence collection using a virtual camera, the VR review platform can automatically form a report of the problems found during review and output a PDF file for customers and design teams to follow up and improve afterwards.
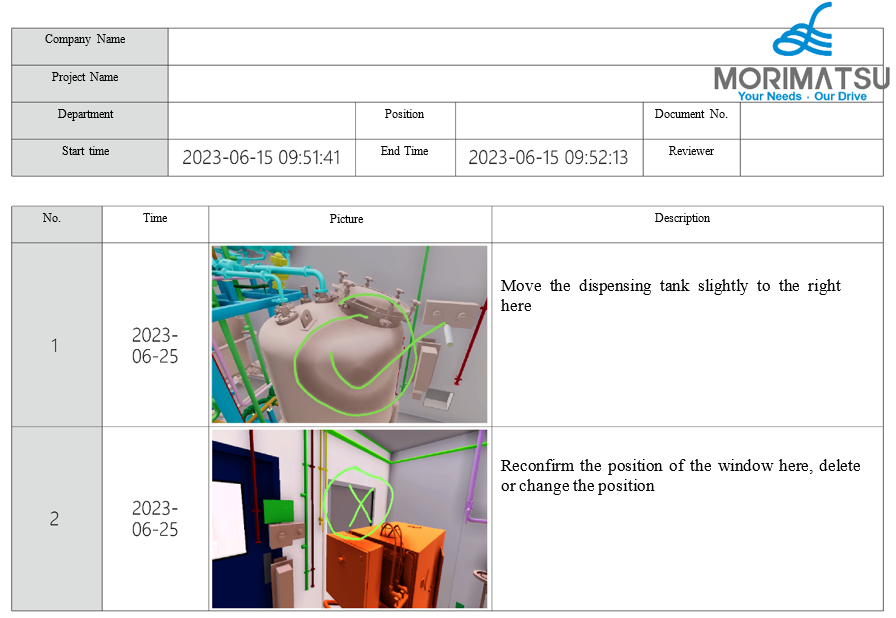
(Review report generation)
Zhang Weiting, Director and General Manager of NCPC, GeneTech Co., Ltd. and related executives participated in the 3D review and spoke highly of Morimatsu's VR review platform.

Morimatsu's VR platform brings a strong sense of reality, and this immersive experience is very rare, which is especially of great help for pharmaceutical plant managers to participate in the pre-design process, especially for those lacking of spatial concept abilities. It is also a good tool to assist us in communicating with Morimatsu's professional 3D design engineers to make well-informed decisions on spatial relationships, thus further improving the design.”
NCPC, GeneTech has always been aiming at "being a strong biopharmaceutical company" and "caring for human health". Morimatsu's VR technology is applied in this “Intelligent Upgrading Project of Vaccine Preparation Production Line” to help NCPC, GeneTech accelerate the R&D and production process for the benefit of human health.
Morimatsu is committed to providing customers with total solutions for engineering design and operation and maintenance based on digital twin technology. By leveraging its expertise in the areas of process production, validation system, and overall factory solutions in the biopharmaceutical industry, Morimatsu takes the lead in practicing the concept of "digital service" in the industry, and builds "digital factories” with its customers, thus providing full life cycle services including design, project delivery, and post-operation and maintenance and building a twin star of engineering and software.
About NCPC, GeneTech Co., Ltd.

Founded in May 1997, NCPC, GeneTech Co., Ltd. is a joint venture between North China Pharmaceutical Group (NCPC) and British Moye Biotechnology Development Co., Ltd. It is currently a wholly-owned subsidiary of North China Pharmaceutical Co., Ltd. and a modernized industrial base for modular biotechnology products in China, mainly engaged in the R&D, production and sales of high-tech, high value-added biotechnology drugs and other pharmaceutical products.
About Morimatsu LifeSciences

Morimatsu LifeSciences is a subsidiary of Morimatsu International Holdings Limited (Morimatsu International, stock code: 2155.HK), covering pharmaceutical, biopharmaceutical, FMCG, cosmetics, electronic chemicals and other industries, provides clients with "core equipment/Machinery + Value added + digitalized intelligence overall Plant Solutions and Service" (" MVP Solutions & Service"), mainly including Shanghai Morimatsu Pharmaceutical Equipment Engineering Co., LTD., Morimatsu (Suzhou) LifeSciences Co., LTD., Shanghai Morimatsu Biotechnology Co., LTD., Shanghai Mori-Biounion Technology Co.,Ltd. and Pharmadule Morimatsu AB., Morimatsu Pharmadule (Singapore) Private Limited as well as their subsidiaries. Morimatsu LifeSciences is focusing on the research and development, manufacturing, and sales of products in related fields.
Forward-Looking Statements
The information in this press release may include some forward-looking statements. Such statements are essentially susceptible to considerable risks and uncertainties. The use of "predicted", "believed", "forecast", "planned" and/or other similar words/phrases in all statements related to our company is to indicate that the statements are forward-looking ones. Our Company undertakes no obligation to constantly revise such predicted statements.
Forward-looking statements are based on our Company management's current perspectives, assumptions, expectations, estimations, predictions and understanding of future affairs at the time of the making of such statements. Such statements are not guarantees of future development and are susceptible to the impact of risks, uncertainties and other factors; some are beyond the control of our Company and unpredictable. Subject to the influence of future changes and development in our business, competition environment, political, economic, legal and social conditions, the actual outcomes may differ significantly from the information contained in the forward-looking statements.