
Search

In July 2023, Morimatsu's single-use system officially obtained the DMF Class III filing number from the FDA, U.S., which will bring the following advantages and conveniences to customers using Morimatsu's single-use system:
1. When marketing applicants for different formulations are using the same Morimatsu DMF filing number, the FDA does not need to conduct multiple reviews, saving review resources;
2. For the applicants using Morimatsu's disposable system, when submitting the registration application information to FDA, they can directly use the DMF registration number, which effectively simplifies the steps of formulation application.
About DMF
A Drug Master File (DMF) is a document submitted to the FDA for review that contains detailed information about the manufacturing facilities, processes, quality control, raw materials and packaging materials used in the manufacture, processing, packaging, storage, and wholesale activities of drugs for human.

Morimatsu’s Total Disposable System Solution
By virtue of professional and complete ISO Class C+A production line, plus a wealth of experience in customer delivery, Morimatsu provides sustainable, safe, stable and customized disposable system solutions for customers.
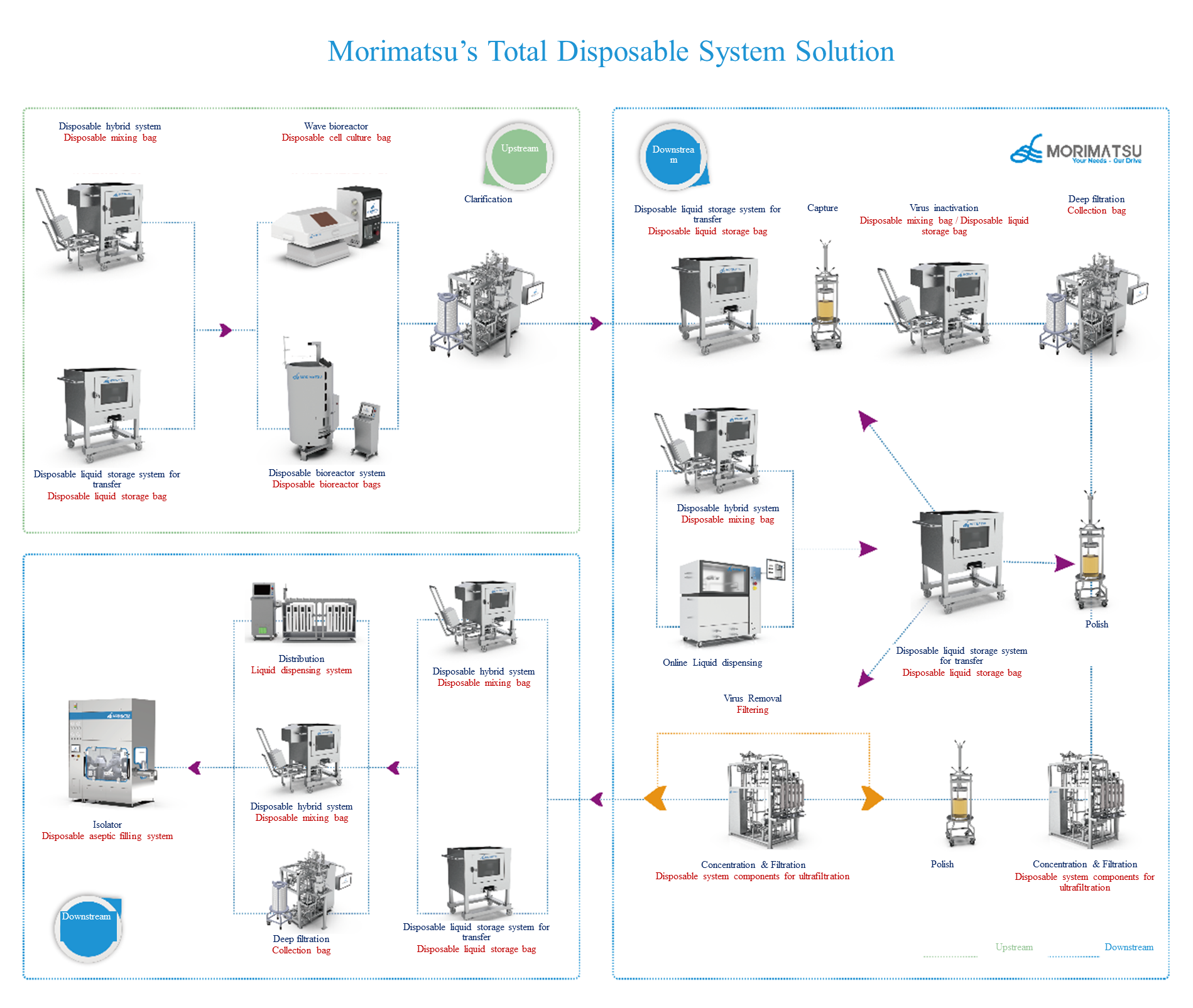
Wave bioreactor

Wave bioreactor systems have been validated globally for a wide range of cell culture and fermentation applications, and it is suitable for laboratory-scale cell culture, as well as process development scale-up and cGMP commercial production. Morimatsu offers pre-sterilized disposable cell culture bags for use with wave bioreactors to provide customers with simple, stable and reliable solution for high-density cell culture technology.
Disposable Bioreactor System

Morimatsu's disposable bioreactor system features advantages of modularity, one-stop solution, and mixing-tank design for bioreactor platform. With specifications ranging from 50L-2000L, the products are designed for scalability and stability, providing the performance and flexibility needed from process development to large-scale biopharmaceutical production for systematic operations such as batch culture, replenishment culture, and perfusion mode.
Disposable liquid storage system for transfer

The storage and transfer of liquids is a crucial part of the entire biotechnology process, and single-use storage bags are widely used in the pharmaceutical process thanks to their simplicity and ease of operation. Morimatsu can provide complete single-use liquid storage solutions based on customer needs. Morimatsu's disposable liquid storage portfolios include 2D liquid storage system, 3D liquid storage system, disposable weighing and feeding bag, and 3D open bag.
Disposable hybrid system

Morimatsu's disposable mixing system consists of 3D mixing bags equipped with 3D containers, including mixing of powder-liquid and liquid-liquid. The volume specification of the disposable 3D mixing bag ranges from 1L-3000L, which is compatible with the mixing equipment systems of different manufacturers in the market, and there are two shapes of mixing bags, that is, round and square, each supporting both closed and open mixing. Based on customer demand, it can be connected to various sensor interfaces to realize online monitoring (temperature, PH, conductivity, etc.), and can be flexibly applied in R&D and pilot commercial production stages, providing customers with safe, stable and efficient disposable mixing systems.
Disposable Aseptic Filling System

Aseptic filling is the last key link of the entire biopharmaceutical preparation production. Morimatsu's disposable aseptic filling system includes disposable liquid storage bag, disposable high level buffer bag, disposable filter, filling line, disposable aseptic connector, disposable disconnector, and so on. Compatible with Morimatsu Isolator or O-RABS, it can realize high precision filling covering grade C to grade A, and the special molding process can reduce the risk of use in pharmaceutical factories.
Morimatsu can provide comprehensive disposable system total solutions according to customers' different requirements, thus meeting the rapidly changing needs of the biopharmaceutical industry.
About Morimatsu LifeSciences
Morimatsu LifeSciences is a subsidiary of Morimatsu International Holdings Limited (Morimatsu International, stock code: 2155.HK), covering pharmaceutical, biopharmaceutical, FMCG, cosmetics, electronic chemicals and other industries, provides clients with "core equipment/Machinery + Value added + digitalized intelligence overall Plant Solutions and Service" (" MVP Solutions & Service"), mainly including Shanghai Morimatsu Pharmaceutical Equipment Engineering Co., LTD., Morimatsu (Suzhou) LifeSciences Co., LTD., Shanghai Morimatsu Biotechnology Co., LTD., Shanghai Mori-Biounion Technology Co.,Ltd. and Pharmadule Morimatsu AB., Morimatsu Pharmadule (Singapore) Private Limited as well as their subsidiaries. Morimatsu LifeSciences is focusing on the research and development, manufacturing, and sales of products in related fields.
From the start in Japan, Morimatsu has grown and today is a diversified multinational corporation with vast experience and professional know-how in fields of process engineering equipment as well as modular engineering solutions. Our company has established long-term collaboration relationships with numerous well-known global corporations. We have companies in Sweden, Italy, US, India, Singapore, Malaysia, together with the large scale operation and manufacturing facilities in Asia. So far, Morimatsu has delivered projects to more than 40 countries/regions in the world, established an excellent industrial reputation in the process.
Forward-Looking Statements
The information in this press release may include some forward-looking statements. Such statements are essentially susceptible to considerable risks and uncertainties. The use of "predicted", "believed", "forecast", "planned" and/or other similar words/phrases in all statements related to our company is to indicate that the statements are forward-looking ones. Our Company undertakes no obligation to constantly revise such predicted statements.
Forward-looking statements are based on our Company management's current perspectives, assumptions, expectations, estimations, predictions and understanding of future affairs at the time of the making of such statements. Such statements are not guarantees of future development and are susceptible to the impact of risks, uncertainties and other factors; some are beyond the control of our Company and unpredictable. Subject to the influence of future changes and development in our business, competition environment, political, economic, legal and social conditions, the actual outcomes may differ significantly from the information contained in the forward-looking statements.